Video Title
DR. FRIEZE:
Hello, my name is Dr. Todd Frieze. I’m an endocrinologist specializing in thyroid disease.
ON SCREEN:
Dr. Todd Frieze
Director of Thyroid Services
Associate Professor of Medicine, Duke University
DR. FRIEZE:
I’m here today to talk about primary hypothyroidism. Primary hypothyroidism occurs when the thyroid gland becomes damaged or inflamed, such that it no longer produces enough thyroid hormone for human body function.
ON SCREEN:
PRIMARY HYPOTHYROIDISM: When the thyroid gland becomes damaged or inflamed and does not produce enough thyroid hormone
DR. FRIEZE:
This is a very common condition. It occurs more in the elderly and is much more common in women than men.
ON SCREEN:
EXPLAINING A PRIMARY HYPOTHYROIDISM
DIAGNOSIS & NTI
DR. FRIEZE:
Primary hypothyroidism is basically diagnosed off of a screening TSH, or thyroid stimulating hormone.
ON SCREEN:
TSH: THYROID STIMULATING HORMONE
DR. FRIEZE:
Family history is very important when talking to these patients because there is a strong predisposition amongst family members in the primary setting to have another family member with hypothyroidism. One of the difficulties of the diagnosis of hypothyroidism is that the signs and symptoms are non-specific to the disease. So, in order to diagnose the disease accurately, given the non-specific symptoms, you must use the TSH level as a screening tool to assist in the family history and signs and symptoms that you have come across. Levothyroxine is the most commonly used treatment in this setting. But therapy has to be individualized for each patient. So, there is no one dose that fits all.
ON SCREEN:
THERE’S NO “ONE DOSE FITS ALL”
DR. FRIEZE:
Levothyroxine is one of the ten NTI drug classes most commonly prescribed. These medications are those where small changes in the dose or blood concentration may lead to serious therapeutic failures and/or adverse drug reactions.
ON SCREEN:
NARROW THERAPEUTIC INDEX MEDICATIONS (NTI): Medications where small changes in the dose or blood concentration may lead to serious therapeutic failures and/or adverse drug reactions.
DR. FRIEZE:
Careful titration of medication and monitoring is required in that setting.
ON SCREEN:
CAREFUL TITRATION & MONITORING IS REQUIRED
DR. FRIEZE:
Overtreatment or undertreatment with levothyroxine may have negative effects on different systems throughout the human body. So, my job as a thyroid specialist in these patients is to make sure my patient is treated and gets to a point of consistency, because it is a lifelong course of medication.
DR. FRIEZE & ON SCREEN:
So, as a reminder, SYNTHROID is indicated for the treatment of hypothyroidism. That is primary, secondary, or tertiary hypothyroidism, either due to congenital or acquired state. It is not indicated for the suppression of benign thyroid nodules, or for non-toxic defuse goiter in iodine-sufficient patients. It is also not indicated for the treatment of hypothyroidism during the recovery phase of subacute thyroiditis. There are safety considerations as SYNTHROID should not be used for treatment of obesity or for weight loss. And SYNTHROID is contraindicated in patients who have uncorrected adrenal insufficiency.
Please see the additional Important Safety Information at the end of this video, including the BOXED WARNING regarding inappropriate treatment for obesity or for weight loss.
DR. FRIEZE:
For healthy adults with hypothyroidism, thyroid hormone replacement should begin at a full replacement dose of 1.6 micrograms per kilogram per day and the doses adjusted or titrated up by 12 ½ to 25 micrograms every 4-6 weeks until the patient is clinically euthyroid and the TSH is normalized.
ON SCREEN:
SYNTHROID is a narrow therapeutic index drug requiring careful titration to avoid the negative effects of overtreatment or undertreatment. In pediatric patients with congenital and acquired hypothyroidism, undertreatment or overtreatment is associated with serious clinical outcomes.
See additional Important Safety Information at the end of this video.
DR. FRIEZE:
There are certain populations where we pay closer attention to thyroid medication dosing. One particular group of these is elderly patients. Another group is those that have underlying cardiac disease or are at risk for atrial fibrillation. And a third is those that have severe, long-standing hypothyroidism.
ON SCREEN:
POPULATIONS TO NOTE:
- Elderly patients
- Patients with underlying cardiac disease
- Patients that have severe, long-standing hypothyroidism
In the elderly and in patients with cardiovascular disease, SYNTHROID should be initiated at lower doses than those recommended in younger individuals or in patients without cardiac disease. Patients with coronary artery disease who are receiving SYNTHROID should be closely monitored for cardiac arrhythmias during surgical procedures.
See additional Important Safety Information at the end of this video.
DR. FRIEZE:
Another special population is pregnant patients. Dosing here should be individualized based on whether the patients were diagnosed with hypothyroidism prior to pregnancy or during the pregnancy.
ON SCREEN:
SYNTHROID Dosing Guidelines for Hypothyroidism in Pregnant Patients [TABLE]
DR. FRIEZE:
If they are newly diagnosed during pregnancy, the dosing is individualized based on the degree of TSH elevation at the time of diagnosis.
ON SCREEN:
SYNTHROID Dosing Guidelines for Hypothyroidism in Pregnant Patients [TABLE]
DR. FRIEZE:
So, getting the patients on the right dose, but maintaining them on the right dose, is also very important. And there are a couple, two, key points that are there.
One is that the peak therapeutic effect of levothyroxine at a given dose may not be obtained for 4-6 weeks after medication is ingested.
ON SCREEN:
1: Peak therapeutic effect may take 4-6 WEEKS
DR. FRIEZE:
The second is that TSH monitoring is generally recommended at 6-8 week intervals until the target TSH is achieved.
ON SCREEN:
2: TSH monitoring is recommended at 6-8 WEEK INTERVALS
DR. FRIEZE:
In addition to testing TSH, sometimes it may be important to run other labs such as a free T4, in addition to the TSH, to ensure you have accurate dosing in the medication.
So, I prescribe Synthroid for several reasons.
The first is that Synthroid has 12 precise dosing options which allows for careful dose titration. This allows us to have an individualized plan for each patient,
ON SCREEN:
[SYNTHROID LOGO] HAS 12 PRECISE DOSING OPTIONS
DR. FRIEZE:
and to adjust the dose based on periodic assessment of the patient's clinical response and their laboratory values. The second is that Synthroid has a history, as physicians have been treating patients with hypothyroidism with Synthroid for over 65 years.
ON SCREEN:
FOR OVER 65 YEARS, PHYSICIANS HAVE BEEN TREATING PATIENTS WITH HYPOTHYROIDISM WITH [SYNTHROID LOGO]
DR. FRIEZE:
Synthroid is widely prescribed. And, as a practicing thyroid specialist, I have extensive clinical experience with using this medication.
ON SCREEN:
TREATING HYPOTHYROIDISM PATIENTS WITH [SYNTHROID LOGO]
DR. FRIEZE:
Once the patient and I have decided that thyroid hormone replacement is necessary, we go over the specific reasons for choosing and writing Synthroid as that replacement therapy. I review with the patients the important issues about how to take the medication, as consistency is really the key message. I talk about the fact that it’s important to take the medication in the same timing and pattern each day, take it on an empty stomach 30-60 minutes before they eat. I also review the safety and risks of taking thyroid medication.
ON SCREEN:
CONSISTENCY IS KEY:
- Take the medication in the same timing and pattern each day
- Take the medication on an empty stomach 30-60 minutes before eating
See additional Important Safety Information at the end of this video.
DR. FRIEZE:
And I, personally, have a patient information sheet that I provide them that goes over some of these important details. For additional information, I direct them to the SYNTHROID website where they can go to the “Starting on SYNTHROID” tab to get that further information they may desire.
I go over with the patient there are treatment goals that are very important in the process. And there are two parts to those treatment goals. One is to obtain their TSH within the normal or goal range. And the other is to improve the clinical symptoms that they’ve been experiencing. Because the long-acting nature of the medication, I do indicate that it may take several months to see an improvement in both of these levels.
ON SCREEN:
TREATMENT GOALS
- Obtain TSH within goal range
- Improve clinical symptoms
NOTE: It make take several months to see an improvement in both of these levels
DR. FRIEZE:
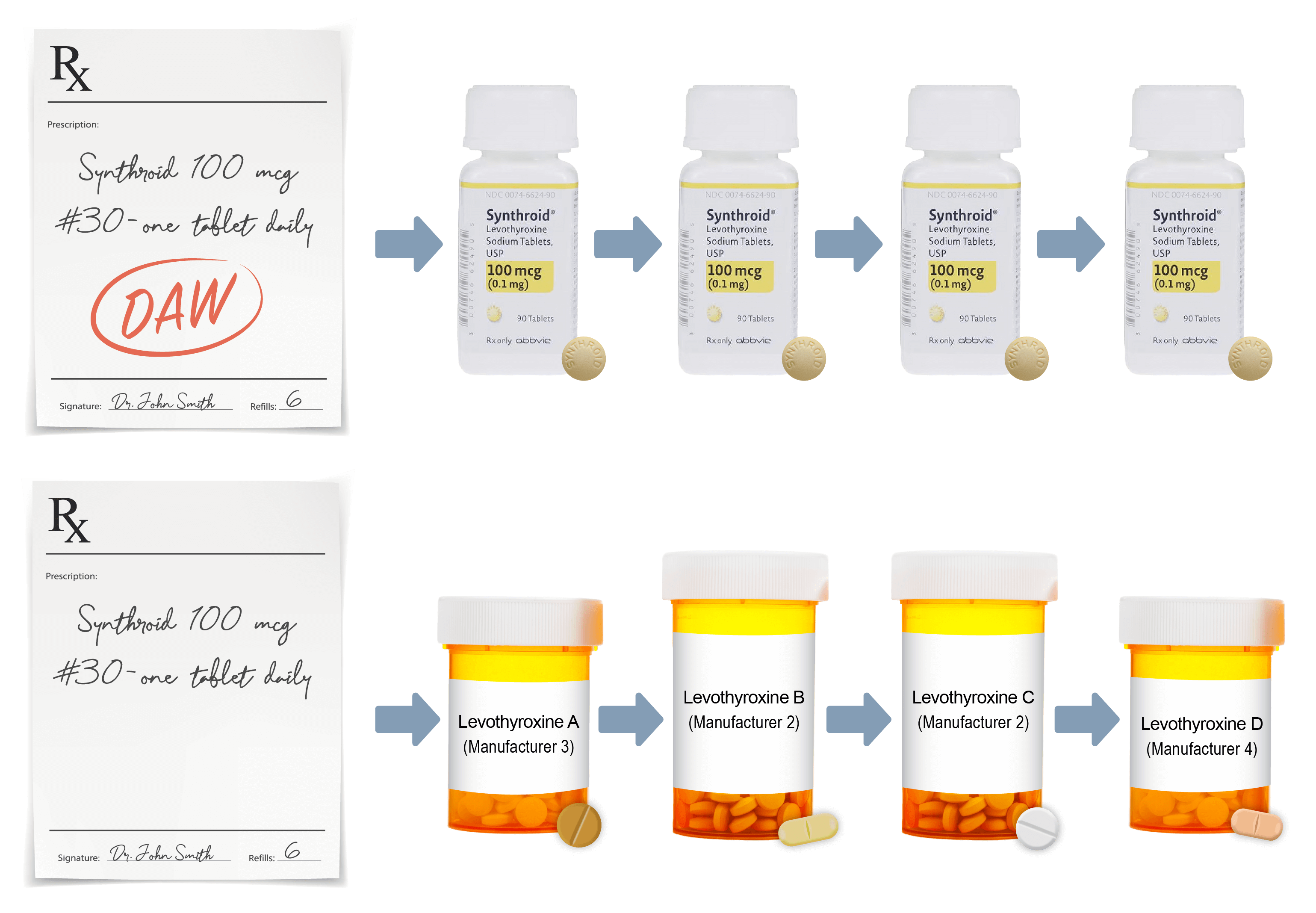
So, it’s not just treating a number and following the TSH, it’s also making sure that the patient’s symptoms have improved. A key part for the patients is to make sure that they’re getting the brand-name SYNTHROID that we have prescribed.
I go over with them the fact that they should check the label at the pharmacy, on the bottle, to make sure it says brand-name SYNTHROID and not generic levothyroxine. And in many cases, to also pop the top off the bottle while they are at the pharmacy counter and look at the pills and make sure they have SYNTHROID embossed on those tablets.
Non-medical substitution – or switching – does occur,
ON SCREEN:
NON-MEDICAL SUBSTITUTION (OR SWITCHING) DOES OCCUR
DR. FRIEZE:
particularly when SYNTHROID is written in a non-protective manner for the state language. Most, but not all, levothyroxine products have been determined to be therapeutically equivalent by the FDA.
ON SCREEN:
The FDA has determined that certain levothyroxine products are interchangeable.
The FDA has determined that drugs that are classified as therapeutically equivalent can be substituted with the full expectation that the substituted product will produce the same clinical effect and safety profile as the reference product.
DR. FRIEZE:
A levothyroxine product that is not therapeutically equivalent with SYNTHROID might not have the exact same effect on the patient’s TSH as SYNTHROID.
ON SCREEN:
NOTE: A levothyroxine product that is not therapeutically equivalent with SYNTHROID might not have the same effects on a patient’s TSH
DR. FRIEZE:
Personally, I have had experiences where I have written for brand-name SYNTHROID and the patients have been dispensed generic levothyroxine or even another branded levothyroxine product. This is a very frustrating issue. It’s very important to make sure that you write “Dispense as written,” or whatever state-specific language, on the prescription when you prescribe the medication.
This is important as 32% of patients who think they are on SYNTHROID are actually not given this because substitutions are made at the pharmacy. 54% of prescriptions for SYNTHROID were not protected with a DAW or state-specific language in one study.
ON SCREEN:
32% of patients were given a substitute for SYNTHROID
Mistaken generic users defined as those who reported taking SYNTHROID but did not have “SYNTHROID” embossed on the pill in a national online survey conducted in 2021 of 1908 adults diagnosed with hypothyroidism and currently taking LT4 products.
54% of prescriptions for SYNTHROID were not protected
34-week prescription audit of 441 pharmacies (average 41 prescriptions per state) totaling 1908 new and renewed SYNTHROID prescriptions between August 1, 2020 and March 23, 2021.
DR. FRIEZE:
The importance of writing “Dispense as Written,” or using the state-specific language for SYNTHROID, is something I discuss with my staff as well. This is very critical, because there are many instances with telephone encounters or other things where patients maybe have prescriptions sent in by my nurse or medical assisting staff, and we need to make sure they understand the importance of this process as well because they are a frontline access to these patients.
This is a very important issue on the initial prescription, but even more importantly to make sure that it occurs with every refill or renewal that occurs, as these are often circumstances where the nursing staff or medical assisting staff are involved in sending those prescriptions in via the electronic records system.
ON SCREEN:
- INITIAL Rx
- REFILL
- RENEWAL
DR. FRIEZE:
Depending on patient circumstances, cost can sometimes be a concern. There are options available to assist the patient with cost concerns.
These include a copay card on the SYNTHROID website
ON SCREEN:
SYNTHROID COPAY CARD
DR. FRIEZE:
and the SYNTHROID Delivers Program.
ON SCREEN:
[SYNTHROID DELIVERS PROGRAM LOGO] and [SYNTHROID LOGO].
DR. FRIEZE:
I have been a big advocate of the SYNTHROID Delivers Program. For one, is its ease of use. I can write the prescription for SYNTHROID and also drop down to the pharmacy and type in SYNTHROID as well, and guarantee that the prescription is sent out to the SYNTHROID pharmacy and know that the patient will be prescribed and dispensed brand-name SYNTHROID only. Some key points that I discuss with the patients during appointments are, one, is that this is an open conversation.
ON SCREEN:
Some key points that I discuss with patients during appointments are:
THIS IS AN OPEN CONVERSATION
DR. FRIEZE:
Patients need to feel comfortable to alert me of any changes in their status in terms of their symptoms. But also specific medical changes that may indicate there may be a need for a dose change or something that could have affected their thyroid hormone levels.
There are reasons for non-scheduled visits. And so, we have certain issues that can come up like patients that become pregnant,
ON SCREEN:
There are reasons for NON-SCHEDULED VISITS
DR. FRIEZE:
they can have changes in the female hormone status, either going on or off a birth control pill, going through menopause. Knowing that patient behaviors and consistency of treatment are key factors in treatment success, I educate patients on the process as they begin treatment.
ON SCREEN:
EDUCATE PATIENTS on the process as they begin treatment
DR. FRIEZE:
One is, I indicate to them that there are certain SYNTHROID programs where the patient can receive additional educational resources that can help them with savings and tips and tools to how to make sure they get the most of their SYNTHROID treatment.
ON SCREEN:
ADDITIONAL EDUCATIONAL RESOURCES ARE AVAILABLE ON SYNTHROID.COM
DR. FRIEZE:
I also go over with patients the fact that they should take the SYNTHROID in the correct way. One is the fact that you can’t take SYNTHROID with coffee or tea. Again, it should be taken on an empty stomach 30-60 minutes before any food or calorie-containing beverages.
ON SCREEN:
[SYNTHROID LOGO]
should be taken on an empty stomach 30-60 minutes before any food or calorie-containing beverages
DR. FRIEZE:
There are certain foods that can interfere with the way SYNTHROID works, particularly we think about high-fiber foods that can interact with the absorption of thyroid medications. There are also certain vitamins and supplements that can interfere with the absorption of SYNTHROID.
This is a key issue for some of the elemental things like calcium and iron, that can interfere with thyroid hormone absorption and thus should be taken approximately 4 hours apart.
ON SCREEN:
Additional information about food or vitamin interactions can be found on the SYNTHROID website.
DR. FRIEZE:
It’s very important to remind the patients, in addition to these factors, that they need to check their pills on a consistent basis. Look to see if the tablets have Synthroid embossed on them to ensure they’re getting the right product.
ON SCREEN:
IN CONCLUSION
DR. FRIEZE:
Well, I’d like to thank you very much for your time today, and I appreciate you joining me and hope you found this session educational and informative. Thanks so much!
ON SCREEN:
For more information, please visit
SYNTHROIDPRO.COM
[SYNTHROID LOGO]
INDICATIONS
Hypothyroidism
SYNTHROID® (levothyroxine sodium) tablets for oral use is an L-thyroxine (T4) indicated in adult and pediatric patients, including neonates, as a replacement therapy in primary (thyroidal), secondary (pituitary), and tertiary (hypothalamic) congenital or acquired hypothyroidism.
Pituitary Thyrotropin (Thyroid Stimulating Hormone, TSH) Suppression
SYNTHROID is indicated in adult and pediatric patients, including neonates, as an adjunct to surgery and radioiodine therapy in the management of thyrotropin-dependent well-differentiated thyroid cancer.
Limitation of Use
SYNTHROID is not indicated for suppression of benign thyroid nodules and nontoxic diffuse goiter in iodine-sufficient patients, as there are no clinical benefits and overtreatment with SYNTHROID may induce hyperthyroidism.
SYNTHROID is not indicated for treatment of hypothyroidism during the recovery phase of subacute thyroiditis.
IMPORTANT SAFETY INFORMATION
WARNING:
Thyroid hormones, including SYNTHROID, either alone or with other therapeutic agents, should not be used for the treatment of obesity or for weight loss. In euthyroid patients, doses within the range of daily hormonal requirements are ineffective for weight reduction. Larger doses may produce serious or even life-threatening manifestations of toxicity, particularly when given in association with sympathomimetic amines such as those used for their anorectic effects.
CONTRAINDICATIONS
- SYNTHROID is contraindicated in patients with uncorrected adrenal insufficiency.
WARNINGS AND PRECAUTIONS
- SYNTHROID has a narrow therapeutic index. Overtreatment or undertreatment with SYNTHROID may have negative effects on growth and development, cardiovascular function, bone metabolism, reproductive function, cognitive function, gastrointestinal function, and glucose and lipid metabolism in adult or pediatric patients. In pediatric patients with congenital and acquired hypothyroidism, undertreatment may adversely affect cognitive development and linear growth, and overtreatment is associated with craniosynostosis and acceleration of bone age. Titrate the dose of SYNTHROID carefully and monitor response to titration to avoid these effects.
- In the elderly and in patients with cardiovascular disease, SYNTHROID should be initiated at lower doses than those recommended in younger individuals or in patients without cardiac disease. If cardiac symptoms develop or worsen, the SYNTHROID dose should be reduced or withheld for one week and restarted at a lower dose.
- Patients with coronary artery disease who are receiving SYNTHROID should be monitored closely during surgical procedures for cardiac arrhythmias. Monitor patients during concomitant administration of SYNTHROID and sympathomimetic agents for signs and symptoms of coronary insufficiency.
- Use of oral thyroid hormone is not recommended in myxedema coma. Products formulated for IV administration should be used to treat myxedema coma.
- Patients with adrenal insufficiency should be treated with replacement glucocorticoids prior to initiating treatment with SYNTHROID. Failure to do so may precipitate an acute adrenal crisis when thyroid hormone therapy is initiated.
- Addition of levothyroxine therapy in patients with diabetes mellitus may worsen glycemic control and result in increased antidiabetic agent or insulin requirements. Carefully monitor glycemic control after starting, changing, or discontinuing SYNTHROID.
- Increased bone resorption and decreased bone mineral density may occur as a result of levothyroxine over-replacement, particularly in postmenopausal women. To mitigate this risk, patients receiving SYNTHROID should be given the minimum dose necessary that achieves the desired response.
ADVERSE REACTIONS
- Adverse reactions associated with SYNTHROID therapy are primarily those of hyperthyroidism due to therapeutic overdosage.
- In pediatric patients receiving levothyroxine therapy, pseudotumor cerebri and slipped capital femoral epiphysis have been reported. Overtreatment may result in craniosynostosis in infants who have not undergone complete closure of the fontanelles, and in premature closure of the epiphyses in pediatric patients still experiencing growth with resultant compromised adult height.
DRUG INTERACTIONS
- Many drugs and some foods affect thyroid hormone pharmacokinetics and metabolism and may alter the therapeutic response to SYNTHROID. In addition, thyroid hormones and thyroid status have varied effects on the pharmacokinetics and actions of other drugs. Administer at least 4 hours before or after drugs that are known to interfere with absorption. Evaluate the need for dose adjustments when regularly administering within one hour of certain foods that may affect absorption. Prescribers should consult appropriate reference sources for additional information on drug or food interactions with SYNTHROID.
USE IN SPECIFIC POPULATIONS
- SYNTHROID should not be discontinued during pregnancy, and hypothyroidism diagnosed during pregnancy should be promptly treated. TSH levels may increase during pregnancy, so TSH should be monitored and SYNTHROID dose adjusted as needed.
Please see Full Prescribing Information on this website.
© 2023 AbbVie. All rights reserved.