Types of patients with hypothyroidism
Some patients with hypothyroidism can be challenging to treat and require special consideration. Use this section to review the cases of Steve, Jennifer, and Diana to see how to effectively manage their hypothyroidism with Synthroid (levothyroxine sodium).

Steve, 50
Recently diagnosed patient*
- Recently diagnosed with hypothyroidism
- Initiating treatment

Jennifer, 32
Pregnant patient*
- Diagnosed with hypothyroidism 3 years ago
- Started and managed on levothyroxine

Diana, 70
Elderly patient*
- Diagnosed with hypothyroidism 10 years ago
- Gastroesophageal reflux disease (GERD) diagnosed 6 months ago
Clinical insights
- Managing hypothyroidism requires frequent monitoring
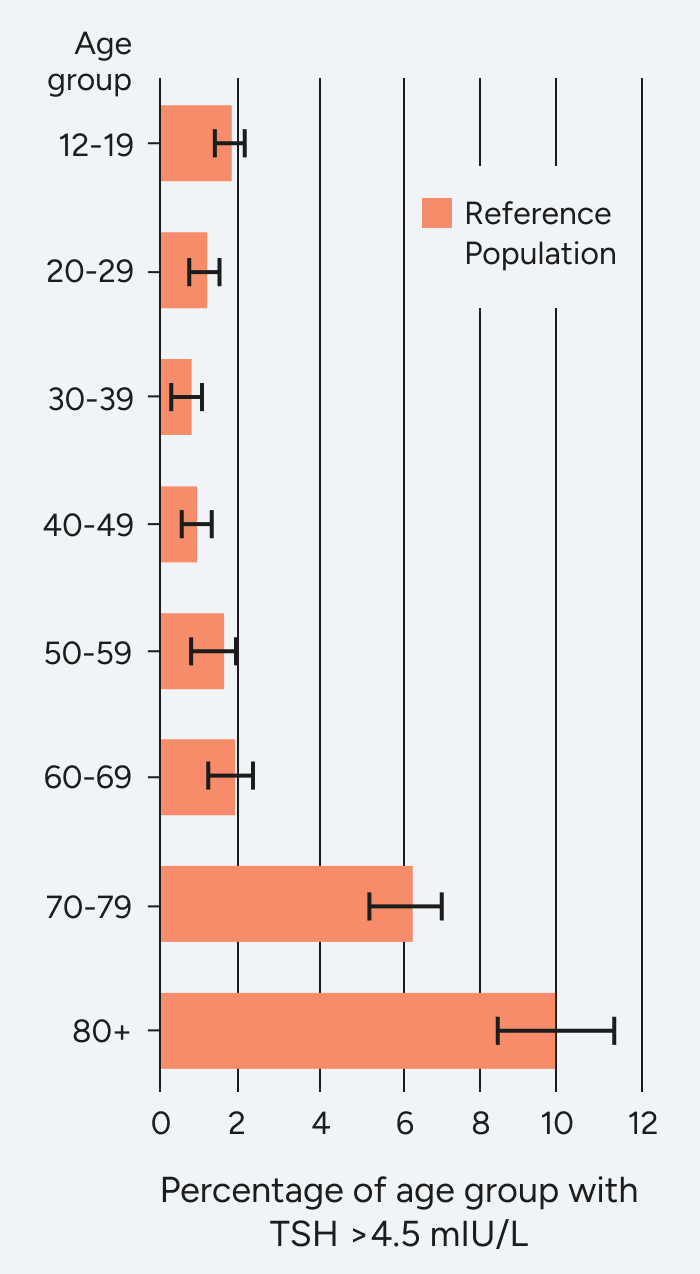
- Study of more than 1500 patients found that 40% of patients being treated for hypothyroidism were not within the normal therapeutic TSH range15
Many patients on thyroid medication struggle to reach optimal TSH levels15,16
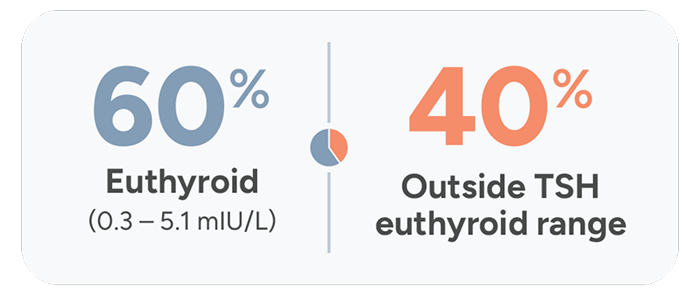
The Colorado Thyroid Disease Prevalence Study
The Colorado Study, a cross-sectional study of patients (N=25,862) at a statewide health fair in 1995, found that only 60% of hypothyroidism patients receiving replacement therapy had normal TSH levels.
25 mcg
orange
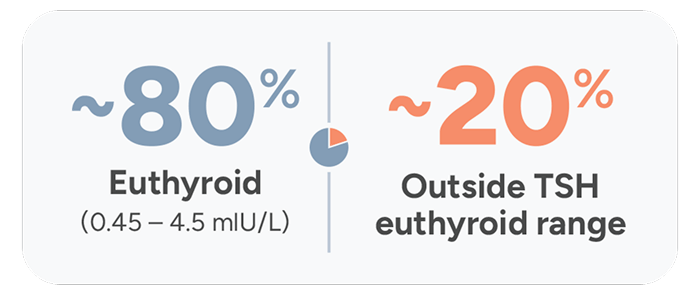
Treatment Adequacy Study
A retrospective Treatment Adequacy study was conducted between 2013-2020 and found that over 20 years later, ~20% of patients taking thyroid medication still do not achieve normal TSH levels.
25 mcg
orange
Overall, 81.1% ([CI: 80.4-81.8]; N=9130) of patients had normal mean TSH levels and 11.7% [CI: 11.1-12.3; N=1312] and 7.3% [CI: 6.8-7.8; N=817] had high and low TSH levels, respectively.
Achieving steady TSH levels and hormonal stability requires precise, individualized dosing, and adequate monitoring is essential in stabilizing TSH levels.2
- Under- or overmedicating can have serious effects2
- With a narrow therapeutic range, even small dose changes in levothyroxine, including Synthroid, can affect TSH levels2
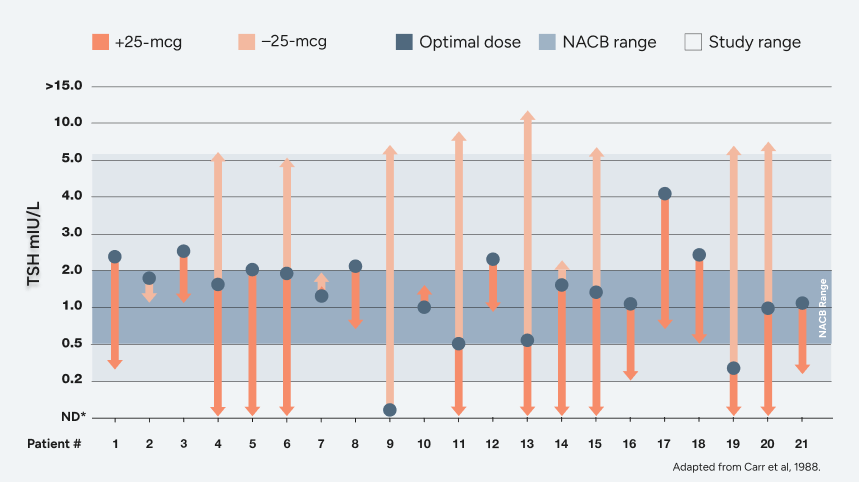
The Carr study: proof that precise dosing matters17
Adapted from a 1988 biochemical study conducted in 21 adult Caucasian patients in the United Kingdom with primary hypothyroidism who were tested on a series of different thyroxine dosages to evaluate effectiveness of measuring TSH levels to monitor thyroid function.
Effect on TSH levels of 25-mcg change in levothyroxine dose17,18
Clinical insights
TSH levels may increase during pregnancy. TSH should be monitored and Synthroid dosage adjusted during pregnancy.1
- 2017 ATA Guidelines state that19:
- Between 50% and 85% of treated hypothyroid women need to increase their levothyroxine dosing during pregnancy
Clinical insights