Some patients with hypothyroidism can be challenging to treat and require special consideration. Use this section to review the cases of Steve, Jennifer, and Diana to see how to effectively manage their hypothyroidism with SYNTHROID (levothyroxine sodium).

Steve, 50
Recently diagnosed patient*
- Recently diagnosed with hypothyroidism
- Initiating treatment

Jennifer, 32
Pregnant patient*
- Diagnosed with hypothyroidism 3 years ago
- Started and managed on levothyroxine

Diana, 70
Elderly patient*
- Diagnosed with hypothyroidism 10 years ago
- Gastroesophageal reflux disease (GERD) diagnosed 6 months ago
*Hypothetical patient profile.

Section Header
- Sed ut perspiciatis unde omnis iste natus error sit voluptatem accusantium doloremque laudantium, totam rem aperiam, eaque ipsa quae ab illo inventore veritatis et quasi architecto beatae vitae dicta sunt explicabo.
- Nemo enim ipsam voluptatem quia voluptas sit aspernatur aut odit aut fugit.
Steve is a 50-year-old man with hypothyroidism.

- 4 months ago, 190-lb patient presented with weight gain of 20 pounds in 1 year. Patient complained of fatigue, dry skin, constipation, cold intolerance, TSH 10.2 mIU/L, BP 150/60, total cholesterol 235 mg/dL; patient was diagnosed with hypothyroidism and started on SYNTHROID 137 mcg
- 8 weeks later, patient’s complaints continued. TSH 4.6 mIU/L; SYNTHROID dose increased to 150 mcg
- Nervousness, agitation, palpitations, and feeling hot for the past few months
- Patient is displaying symptoms of being overreplaced with current dose of SYNTHROID of 150 mcg; TSH test confirmed
- Decrease dose of SYNTHROID
- Recheck TSH levels in 4-6 weeks
*Hypothetical patient profile.
CLINICAL INSIGHT
- Managing hypothyroidism requires frequent monitoring
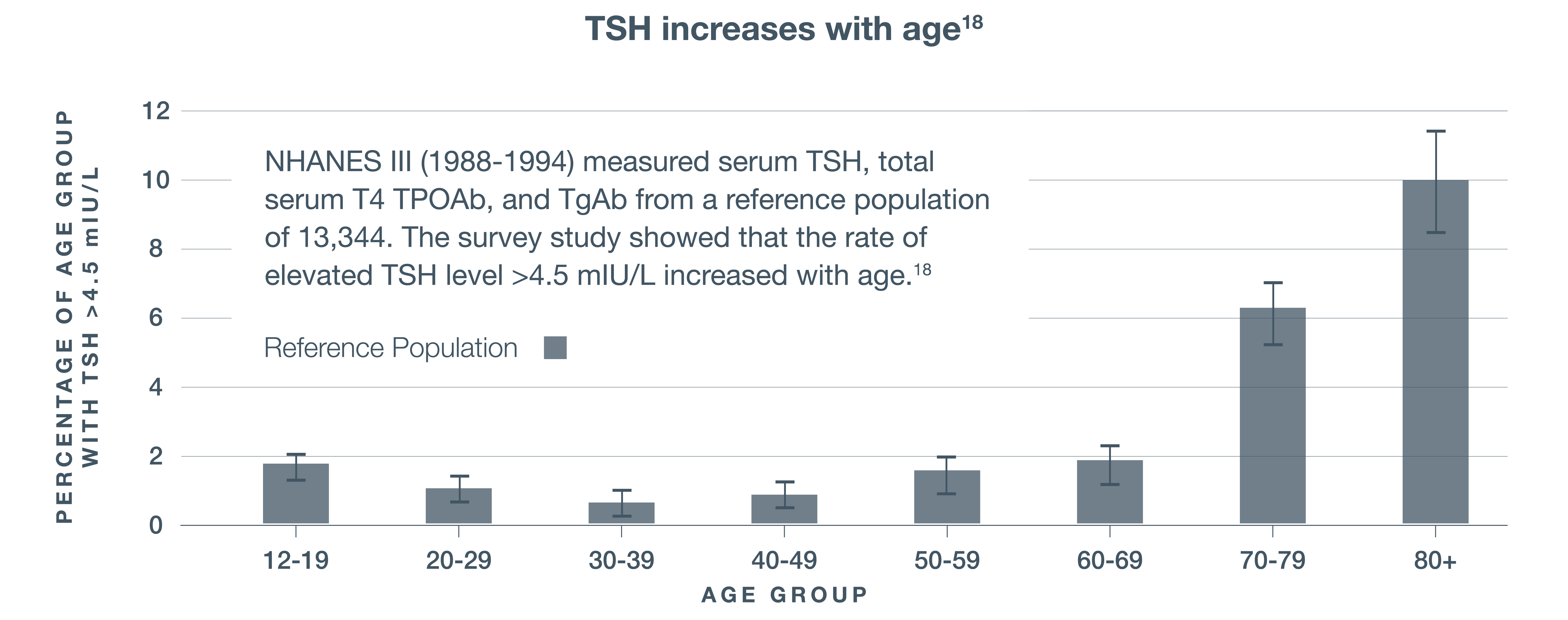
Study of more than 1500 patients found that 40% of patients being treated for hypothyroidism were not within the normal therapeutic TSH range15
COLORADO THYROID DISEASE PREVALENCE STUDY15
60%
Euthyroid†
40%
Outside of therapeutic TSH range



(N=1525)
Therapeutic TSH range: 0.3-5.1 mIU/L
†Euthyroid=normal thyroid function.
Achieving steady TSH levels and hormonal stability requires precise, individualized dosing, and adequate monitoring is essential in stabilizing TSH levels.2
- Under- or overmedicating can have serious effects2
- With a narrow therapeutic range, even small dose changes in levothyroxine, including SYNTHROID, can affect TSH levels2
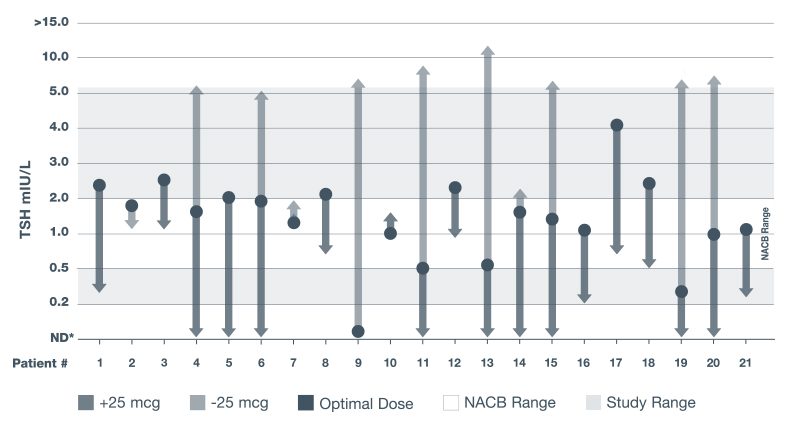
THE CARR STUDY: PROOF THAT PRECISE DOSING MATTERS16
Adapted from a 1988 biochemical study conducted in 21 adult Caucasian patients in the United Kingdom with primary hypothyroidism who were tested on a series of different thyroxine dosages to evaluate effectiveness of measuring TSH levels to monitor thyroid function.16
Following a 25-mcg dose change of levothyroxine, most patients had changes in TSH levels.

Section Header
- Sed ut perspiciatis unde omnis iste natus error sit voluptatem accusantium doloremque laudantium, totam rem aperiam, eaque ipsa quae ab illo inventore veritatis et quasi architecto beatae vitae dicta sunt explicabo.
- Nemo enim ipsam voluptatem quia voluptas sit aspernatur aut odit aut fugit.
Jennifer is a 32-year-old woman in her first trimester of pregnancy with
hypothyroidism that is managed on SYNTHROID.

- Diagnosed with hypothyroidism 3 years ago
- Started and managed on 50 mcg SYNTHROID
- Weight fluctuation
- Appendectomy
- Fatigue and facial puffiness
- Patient is displaying symptoms of hypothyroidism due to subtherapeutic dosing of SYNTHROID; TSH test confirmed
- Increase dosage of SYNTHROID
- Recheck TSH levels in 4 weeks
*Hypothetical patient profile.
CLINICAL INSIGHT
TSH levels may increase during pregnancy. TSH should be monitored and SYNTHROID dosage adjusted during pregnancy.
- 2017 ATA Guidelines state that17:
Between 50% and 85% of treated hypothyroid women need to increase their levothyroxine dosing during pregnancy
ELDERLY PATIENT*

Section Header
- Sed ut perspiciatis unde omnis iste natus error sit voluptatem accusantium doloremque laudantium, totam rem aperiam, eaque ipsa quae ab illo inventore veritatis et quasi architecto beatae vitae dicta sunt explicabo.
- Nemo enim ipsam voluptatem quia voluptas sit aspernatur aut odit aut fugit.
Diana is a 70-year-old woman who has a history of
hypothyroidism that has been managed on 112 mcg of SYNTHROID for the past 5 years.

- Hypothyroidism diagnosed 10 years ago
- Gastroesophageal reflux disease (GERD) diagnosed 6 months ago
- Enlarged thyroid
- Increasing hair loss, fatigue, chronic constipation for the past few months
- Patient is displaying symptoms of hypothyroidism (TSH test confirmed), most likely due to the initiation of a PPI and the concurrent use of daily fiber supplements
- Counseled patient to take SYNTHROID on an empty stomach and at least 4 hours before or after other medications
- Recheck TSH levels in 6-8 weeks
*Hypothetical patient profile.
CLINICAL INSIGHT
Gastric acidity is essential for adequate absorption of levothyroxine; therefore, administration of a PPI may affect the intragastric pH, which can reduce absorption
Levothyroxine should be taken on an empty stomach and at least 4 hours before or after medications known to interfere with absorption
Dietary fiber may bind and decrease the absorption of levothyroxine from the gastrointestinal tract